A Wisconsin company recently recalled 5-ounce packages of alfalfa sprouts due to concerns that the food could be contaminated with listeria...
Vous n'êtes pas connecté
- English
- Français
- عربي
- Español
- Deutsch
- Português
- русский язык
- Català
- Italiano
- Nederlands, Vlaams
- Norsk
- فارسی
- বাংলা
- اردو
- Azərbaycan dili
- Bahasa Indonesia
- Հայերեն
- Ελληνικά
- Bosanski jezik
- українська мова
- Íslenska
- Türkmen, Түркмен
- Türkçe
- Shqip
- Eesti keel
- magyar
- Қазақ тілі
- Kalaallisut ; kalaallit oqaasii
- Lietuvių kalba
- Latviešu valoda
- македонски јазик
- Монгол
- Bahasa Melayu ; بهاس ملايو
- ဗမာစာ
- Slovenščina
- тоҷикӣ ; toğikī ; تاجیکی
- ไทย
- O'zbek ; Ўзбек ; أۇزبېك
- Tiếng Việt
- ភាសាខ្មែរ
- རྫོང་ཁ
- Soomaaliga ; af Soomaali
Rubriques :
 Maroc - NEWSDAY.CO.TT - A la Une - Hier 16:05
Maroc - NEWSDAY.CO.TT - A la Une - Hier 16:05
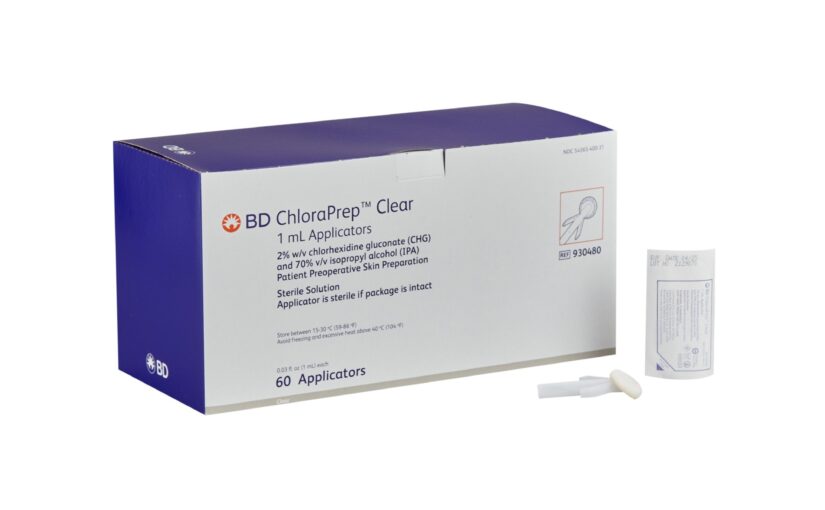
Ministry: Surgery prep applicator voluntarily recalled
The Ministry of Health says there has been a voluntary recall of one lot of ChloraPrep Clear 1 mL Applicator. These applicators are used for the preparation of a patient’s skin prior to surgery and helps reduce bacteria that can potentially cause skin infections. In a release, the ministry said the recall, as stated in a release from BD (Becton, Dickinson and Co), is “due to fungal contamination under certain environmental conditions allowing the growth of Aspergillus penicillioides." The Aspergillus penicillioides within the packaging can contaminate the surface of the applicator and/or gloved hands of the healthcare professional and then consequently the sterile field. Since the applicator is used for site preparation prior to an invasive procedure, a contaminated applicator can result in direct inoculation of the fungus into tissues. Contamination of skin preparation products with Aspergillus penicillioides may lead to serious systemic infection, sepsis, illness, and death. The affected lot of ChloraPrep Clear 1 mL Applicators can be identified by locating the referenced catalog number - 930480 and lot number – 3200240, on the lidding of the product package and on the side of the individual shelf box and shipper box. BD’s release further stated, “This lot was distributed globally beginning in September 2023. To date, there have been no reported customer complaints or adverse events associated with this issue”. While the recalled products are not registered for use in TT, the ministry said, out of an abundance of caution it advises people who may be in possession of the identified lot of ChloraPrep Clear 1 mL Applicators to discontinue use immediately. Additional information can be obtained by contacting the Office of the Director of the Chemistry, Food and Drugs Division via email at cfdd@health.gov.tt or phone at 217-4664 ext. 13101. The post Ministry: Surgery prep applicator voluntarily recalled appeared first on Trinidad and Tobago Newsday.
Articles similaires
Health Ministry issues voluntary recall of canned tuna
The Ministry of Health's Chemistry, Food and Drugs Division has advised of a voluntary recall notice for select lots of canned tuna products sold...
Possible Listeria Contamination Prompts Recall of 2 Million Baked Goods
MONDAY, Feb. 10, 2025 -- About 2 million baked goods have been recalled over concerns of potential contamination with Listeria monocytogenes,...
Social Development Ministry denies links with foundation
The Ministry of Social Development and Family Services has denied any affiliation with a foundation which allegedly claimed to provide social...
Some MEC snowsuits recalled due to mould
Mountain Equipment Company is recalling two types of children's snowsuits due to concern about mould contamination. The MEC Toaster and Toaster...
Some MEC snowsuits recalled due to mould
Mountain Equipment Company is recalling two types of children's snowsuits due to concern about mould contamination. The MEC Toaster and Toaster...
ICU Medical Issues Nationwide Recall of Potassium Chloride Injection, 20 mEq and Potassium Chloride Injection, 10 mEq Due to Mislabeling
Audience: Consumer, Health Professional, Pharmacy February 13, 2025 LAKE FOREST, Illinois – ICU Medical, Inc. is voluntarily recalling one lot...
Some MEC snowsuits recalled due to mould
Mountain Equipment Company is recalling two types of children's snowsuits due to concern about mould contamination. The MEC Toaster and Toaster...
Some MEC snowsuits recalled due to mould
Mountain Equipment Company is recalling two types of children's snowsuits due to concern about mould contamination. The MEC Toaster and Toaster...
Some MEC snowsuits recalled due to mould
Mountain Equipment Company is recalling two types of children's snowsuits due to concern about mould contamination. The MEC Toaster and Toaster...
Les derniers communiqués
-
Aucun élément